|
|
|
 Taj.Products Taj.Products |
|
|
 Taj Brands
Taj Brands |
 |
|
 |
|
 |
|
Therapeutic Index |
|
 |
|
Each
Tablet contains:
clopidogrel bisulfate 75 mg tablets
COMPOSITION :
Plogryl...75mg
Each film-coated tablet contains:
Clopidogrel (as bisulfate) ...... 75 mg
PLOGRYL® (clopidogrel bisulfate): Help
prevent Clot formation for ...
PLOGRYL, proven to help protect against
future heart attack or stroke. Click for
safety and prescribing information.
PLOGRYL helps protect you
against a future heart attack or stroke
If you have had one clot-related event-a
heart attack, heart-related chest pain,
or stroke -you are at higher risk of
another clot-related event. If you have
been diagnosed with Peripheral Artery
Disease (P.A.D., known as poor
circulation in the legs), you are also
at higher risk of a heart attack or
stroke.
PLOGRYL helps keep platelets in the
blood from sticking together and forming
clots- the direct cause of most heart
attacks and strokes. With its
effectiveness proven and safety profile
supported by 4 large clinical studies
with 81,000 patients, PLOGRYL is the #1
prescription
antiplatelet medicine.
Remember, your doctor is the single best
source of information regarding your
health.
Please consult your doctor if you have
any questions about your health or your
medicine.
|
PLOGRYL® |
Prescribing Information |
|
clopidogrel bisulfate tablets |
Rx only |
DESCRIPTION :
PLOGRYL (clopidogrel bisulfate) is
an inhibitor of ADP-induced platelet
aggregation acting by direct inhibition
of adenosine diphosphate (ADP) binding
to its receptor and of the subsequent
ADP-mediated activation of the
glycoprotein GPIIb/IIIa complex.
Chemically it is methyl
(+)-(S)-α-(2-chlorophenyl)-6,7-dihydrothieno[3,2-c]pyridine-5(4H)-acetate
sulfate (1:1). The empirical formula of
clopidogrel bisulfate is
C16H16ClNO2S•H2SO4 and its molecular
weight is 419.9.
The structural formula is as follows:
|
|
 |
|
Clopidogrel bisulfate is a white to
off-white powder. It is practically
insoluble in water at neutral pH but
freely soluble at pH 1. It also
dissolves freely in methanol, dissolves
sparingly in methylene chloride, and is
practically insoluble in ethyl ether. It
has a specific optical rotation of about
+56°.
PLOGRYL for oral administration is
provided as either pink, round,
biconvex, debossed, film-coated tablets
containing 97.875 mg of clopidogrel
bisulfate which is the molar equivalent
of 75 mg of clopidogrel base or pink,
oblong, debossed film-coated tablets
containing 391.5 mg of clopidogrel
bisulfate which is the molar equivalent
of 300 mg of clopidogrel base.
Each tablet contains hydrogenated castor
oil, hydroxypropylcellulose, mannitol,
microcrystalline cellulose and
polyethylene glycol 6000 as inactive
ingredients. The pink film coating
contains ferric oxide, hypromellose
2910, lactose monohydrate, titanium
dioxide and triacetin. The tablets are
polished with Carnauba wax.
CLINICAL PHARMACOLOGY :
Mechanism of Action and
Pharmacodynamic Properties
Clopidogrel is a prodrug, one of whose
metabolites is an inhibitor of platelet
aggregation. A variety of drugs that
inhibit platelet function have been
shown to decrease morbid events in
people with established cardiovascular
atherosclerotic disease as evidenced by
stroke or transient ischemic attacks,
myocardial infarction, unstable angina
or the need for vascular bypass or
angioplasty. This indicates that
platelets participate in the initiation
and/or evolution of these events and
that inhibiting platelet function can
reduce the event rate.
Clopidogrel must be metabolized by
CYP450 enzymes to produce the active
metabolite that inhibits platelet
aggregation. The active metabolite of
clopidogrel selectively inhibits the
binding of adenosine diphosphate (ADP)
to its platelet P2Y12 receptor and the
subsequent ADP-mediated activation of
the glycoprotein GPIIb/IIIa complex,
thereby
inhibiting platelet aggregation. This
action is irreversible. Consequently,
platelets exposed to clopidogrel's
active metabolite are affected for the
remainder of their lifespan (about 7 to
10 days). Platelet aggregation induced
by agonists other than ADP is also
inhibited by blocking the amplification
of platelet activation by released ADP.
Because the active metabolite is formed
by CYP450 enzymes, some of which are
polymorphic or subject to inhibition by
other drugs, not all patients will have
adequate platelet inhibition.
Dose dependent inhibition of platelet
aggregation can be seen 2 hours after
single oral doses of PLOGRYL. Repeated
doses of 75 mg PLOGRYL per day inhibit
ADP-induced platelet aggregation on the
first day, and inhibition reaches steady
state between Day 3 and Day 7. At steady
state, the average inhibition level
observed with a dose of 75 mg PLOGRYL
per day was between 40% and 60%.
Platelet aggregation and bleeding time
gradually return to baseline values
after treatment is discontinued,
generally in about 5 days.
Pharmacokinetics
Absorption
After single and repeated oral doses of
75 mg per day, clopidogrel is rapidly
absorbed. Mean peak plasma levels of
unchanged clopidogrel (approximately
2.2-2.5 ng/mL after a single 75-mg oral
dose) occurred approximately 45 minutes
after dosing. Absorption is at least
50%, based on urinary excretion of
clopidogrel metabolites.
Effect of Food
The effect of food on the
bioavailability of the parent compound
or active metabolite is currently not
known.
Distribution
Clopidogrel and the main circulating
inactive metabolite bind reversibly in
vitro to human plasma proteins (98% and
94%, respectively). The binding is
nonsaturable in vitro up to a
concentration of 100 mcg/mL.
Metabolism
Clopidogrel is extensively metabolized
by the liver. In vitro and in vivo,
clopidogrel is metabolized according to
two main metabolic pathways: one
mediated by esterases and leading to
hydrolysis into its inactive carboxylic
acid derivative (85% of circulating
metabolites), and one mediated by
multiple cytochromes P450. Cytochromes
first oxidize clopidogrel to a
2-oxo-clopidogrel intermediate
metabolite. Subsequent metabolism of the
2-oxo-clopidogrel intermediate
metabolite results in formation of the
active metabolite, a thiol derivative of
clopidogrel. In vitro, this metabolic
pathway is mediated by CYP3A4, CYP2C19,
CYP1A2 and CYP2B6. The active thiol
metabolite, which has been isolated in
vitro, binds rapidly and irreversibly to
platelet receptors, thus inhibiting
platelet aggregation.
Elimination
Following an oral dose of
14C-labeled clopidogrel in humans,
approximately 50% of total radioactivity
was excreted in urine and approximately
46% in feces over the 5 days
post-dosing. After a single, oral dose
of 75 mg, clopidogrel has a half-life of
approximately 6 hours. The elimination
half-life of the inactive acid
metabolite was 8 hours after single and
repeated administration. Covalent
binding to platelets accounted for 2% of
radiolabel with a half-life of 11 days.
In plasma and urine, the glucuronide of
the carboxylic acid derivative is also
observed.
Pharmacogenetics
Several polymorphic CYP450 enzymes
activate clopidogrel. CYP2C19 is
involved in the formation of both the
active metabolite and the
2-oxo-clopidogrel intermediate
metabolite. Clopidogrel active
metabolite pharmacokinetics and
antiplatelet effects, as measured by ex
vivo platelet aggregation assays, differ
according to CYP2C19 genotype. The
CYP2C19*1 allele corresponds to fully
functional metabolism while the
CYP2C19*2 and CYP2C19*3 alleles
correspond to reduced metabolism. The
CYP2C19*2 and CYP2C19*3 alleles account
for 85% of reduced function alleles in
whites and 99% in Asians. Other alleles
associated with reduced metabolism
include CYP2C19*4, *5, *6, *7, and *8,
but these are less frequent in the
general population. Published
frequencies for the common CYP2C19
phenotypes and genotypes are listed in
the table below.
|
|
Table 1 - CYP2C19 Phenotype and
Genotype Frequency |
| |
Frequency (%) |
| |
White
(n=1356) |
Black (n=966) |
Chinese (n=573) |
|
Xie, et al. Annu Rev Pharmacol
Toxicol 2001; 41: 815-50 |
|
Extensive metabolism: CYP2C19*1/*1 |
74 |
66 |
38 |
|
Intermediate metabolism:
CYP2C19*1/*2 or *1/*3 |
26 |
29 |
50 |
|
Poor
metabolism: CYP2C19*2/*2, *2/*3 or
*3/*3 |
2 |
4 |
14 |
|
|
To date, the impact of CYP2C19 genotype
on the pharmacokinetics of clopidogrel's
active metabolite has been evaluated in
227 subjects from 7 reported studies.
Reduced CYP2C19 metabolism in
intermediate and poor metabolizers
decreased the Cmax and AUC of the active
metabolite by 30-50% following 300- or
600 mg loading doses and 75 mg
maintenance doses. Lower active
metabolite exposure results in less
platelet inhibition or higher residual
platelet reactivity. To date, diminished
antiplatelet responses to clopidogrel
have been described for intermediate and
poor metabolizers in 21 reported studies
involving 4,520 subjects. The relative
difference in antiplatelet response
between genotype groups varies across
studies depending on the method used to
evaluate response, but is typically
greater than 30%.
The association between CYP2C19 genotype
and clopidogrel treatment outcome was
evaluated in 2 post-hoc clinical trial
analyses (substudies of CLARITY-TIMI 281
[n=465] and TRITON-TIMI 382 [n=1,477])
and 5 cohort studies (total n=6,489). In
CLARITY-TIMI 28 and one of the cohort
studies (n=765; Trenk3), cardiovascular
event rates did not differ significantly
by genotype. In TRITON-TIMI 38 and 3 of
the cohort studies (n= 3,516; Collet,4
Sibbing,5 Giusti6), patients with an
impaired metabolizer status
(intermediate and poor combined) had a
higher rate of cardiovascular events
(death, myocardial infarction, and
stroke) or stent thrombosis compared to
extensive metabolizers. In the fifth
cohort study (n=2,208; Simon7), the
increased event rate was observed only
in poor metabolizers.
Pharmacogenetic testing can identify
genotypes associated with variability in
CYP2C19 activity.
There may be genetic variants of
other CYP450 enzymes with effects on the
ability to form clopidogrel's active
metabolite.
1.Mega JL, Thakuria JV, Cannon
CP, Sabatine MS. Sequence variations in
CYP metabolism genes and cardiovascular
outcomes following treatment with
clopidogrel: insights from the CLARITY-TIMI
28 genomic study. 2008; ACC Meeting
Abstract
2.Mega et al. Cytochrome P-450
polymorphisms and response to
clopidogrel. N Engl J Med 2009;
360:354-62
3.Trenk et al. Cytochrome P450
2C19 681G>A polymorphism and high on-clopidogrel
platelet reactivity associated with
adverse 1-year clinical outcome of
elective percutaneous coronary
intervention with drug-eluting or
bare-metal stents. J Am Coll Cardiol
2008; 51, 20: 1952
4.Collet JP et al. Cytochrome
P450 2C19 polymorphism in young patients
treated with clopidogrel after
myocardial infarction: a cohort study.
The Lancet 2009; 373: 309-317
5.Sibbing D et al. Cytochrome
P450 2C19 loss-of-function polymorphism
and stent thrombosis following
percutaneous coronary intervention. Eur
Heart J 2009:1-7
6.Giusti et al. Relation of
cytochrome P450 2C19 loss-of-function
polymorphism to occurrence of
drug-eluting coronary stent thrombosis.
Am J Cardiol 2009; 103:806–811
7.Simon et al. Genetic
determinants of response to clopidogrel
and cardiovascular events. N Engl J Med
2009; 360(4):363-75
Special Populations :
The pharmacokinetics of clopidogrel's
active metabolite is not known in these
special populations.
Geriatric Patients :
In elderly (≥75 years) volunteers
compared to young healthy volunteers,
there were no differences in platelet
aggregation and bleeding time. No dosage
adjustment is needed for the elderly.
Renally-Impaired Patients :
After repeated doses of 75 mg PLOGRYL
per day in patients with severe renal
impairment (creatinine clearance from 5
to 15 mL/min), inhibition of ADP-induced
platelet aggregation was lower (25%)
than that observed in healthy
volunteers, however, the prolongation of
bleeding time was similar to healthy
volunteers receiving 75 mg of PLOGRYL
per day.
Hepatically-Impaired Patients
After repeated doses of 75 mg PLOGRYL
per day for 10 days in patients with
severe hepatic impairment, inhibition of
ADP-induced platelet aggregation was
similar to that observed in healthy
subjects. The mean bleeding time
prolongation was also similar in the two
groups.
Gender
In a small study comparing men and
women, less inhibition of ADP-induced
platelet aggregation was observed in
women, but there was no difference in
prolongation of bleeding time. In the
large, controlled clinical study (Clopidogrel
vs. Aspirin in Patients at Risk of
Ischemic Events; CAPRIE), the incidence
of clinical outcome events, other
adverse clinical events, and abnormal
clinical laboratory parameters was
similar in men and women.
Race
The prevalence of CYP2C19 alleles that
result in intermediate and poor CYP2C19
metabolism differs according to
race/ethnicity (see CLINICAL
PHARMACOLOGY: Pharmacogenetics).
CLINICAL STUDIES :
The clinical evidence for the efficacy
of PLOGRYL is derived from four
double-blind trials involving 81,090
patients: the CAPRIE study (Clopidogrel
vs. Aspirin in Patients at Risk of
Ischemic Events), a comparison of
PLOGRYL to aspirin, and the CURE (Clopidogrel
in Unstable Angina to Prevent Recurrent
Ischemic Events), the COMMIT/CCS-2 (Clopidogrel
and Metoprolol in Myocardial Infarction
Trial / Second Chinese Cardiac Study)
studies comparing PLOGRYL to placebo,
both given in combination with aspirin
and other standard therapy and CLARITY-TIMI
28 (Clopidogrel as Adjunctive
Reperfusion Therapy – Thrombolysis in
Myocardial Infarction).
Recent Myocardial Infarction (MI),
Recent Stroke or Established Peripheral
Arterial Disease
The CAPRIE trial was a 19,185-patient,
304-center, international, randomized,
double-blind, parallel-group study
comparing PLOGRYL (75 mg daily) to
aspirin (325 mg daily). The patients
randomized had: 1) recent histories of
myocardial infarction (within 35 days);
2) recent histories of ischemic stroke
(within 6 months) with at least a week
of residual neurological signs; or 3)
objectively established peripheral
arterial disease. Patients received
randomized treatment for an average of
1.6 years (maximum of 3 years).
The trial's primary outcome was the time
to first occurrence of new ischemic
stroke (fatal or not), new myocardial
infarction (fatal or not), or other
vascular death. Deaths not easily
attributable to nonvascular causes were
all classified as vascular.
|
|
Table 2: Outcome Events in the
CAPRIE Primary Analysis |
|
Patients |
Plogryl
9599 |
Aspirin9586 |
|
IS (fatal or not) |
438 (4.6%) |
461 (4.8%) |
|
MI (fatal or not) |
275 (2.9%) |
333 (3.5%) |
|
Other vascular death |
226 (2.4%) |
226 (2.4%) |
|
Total |
939 (9.8%) |
1020 (10.6% |
|
|
As shown in the table, PLOGRYL (clopidogrel
bisulfate) was associated with a lower
incidence of outcome events of every
kind. The overall risk reduction (9.8%
vs. 10.6%) was 8.7%, P=0.045. Similar
results were obtained when all-cause
mortality and all-cause strokes were
counted instead of vascular mortality
and ischemic strokes (risk reduction
6.9%). In patients who survived an
on-study stroke or myocardial
infarction, the incidence of subsequent
events was again lower in the PLOGRYL
group.
The curves showing the overall event
rate are shown in Figure 1. The event
curves separated early and continued to
diverge over the 3-year follow-up
period. |
Figure 1: Fatal or Non-Fatal Vascular
Events in the CAPRIE Study
|
|
 |
|
Although the statistical significance
favoring PLOGRYL over aspirin was
marginal (P=0.045), and represents the
result of a single trial that has not
been replicated, the comparator drug,
aspirin, is itself effective (vs.
placebo) in reducing cardiovascular
events in patients with recent
myocardial infarction or stroke. Thus,
the difference between PLOGRYL and
placebo, although not measured directly,
is substantial.
The CAPRIE trial included a population
that was randomized on the basis of 3
entry criteria. The efficacy of PLOGRYL
relative to aspirin was heterogeneous
across these randomized subgroups
(P=0.043). It is not clear whether this
difference is real or a chance
occurrence. Although the CAPRIE trial
was not designed to evaluate the
relative benefit of PLOGRYL over aspirin
in the individual patient subgroups, the
benefit appeared to be strongest in
patients who were enrolled because of
peripheral vascular disease (especially
those who also had a history of
myocardial infarction) and weaker in
stroke patients. In patients who were
enrolled in the trial on the sole basis
of a recent myocardial infarction,
PLOGRYL was not numerically superior to
aspirin.
In the meta-analyses of studies of
aspirin vs. placebo in patients similar
to those in CAPRIE, aspirin was
associated with a reduced incidence of
thrombotic events. There was a
suggestion of heterogeneity in these
studies too, with the effect strongest
in patients with a history of myocardial
infarction, weaker in patients with a
history of stroke, and not discernible
in patients with a history of peripheral
vascular disease. With respect to the
inferred comparison of PLOGRYL to
placebo, there is no indication of
heterogeneity
Acute Coronary Syndrome
The CURE study included 12,562 patients
with acute coronary syndrome without ST
segment elevation (unstable angina or
non-Q-wave myocardial infarction) and
presenting within 24 hours of onset of
the most recent episode of chest pain or
symptoms consistent with ischemia.
Patients were required to have either
ECG changes compatible with new ischemia
(without ST segment elevation) or
elevated cardiac enzymes or troponin I
or T to at least twice the upper limit
of normal. The patient population was
largely Caucasian (82%) and included 38%
women, and 52% patients ≥65 years of
age.
Patients were randomized to receive
PLOGRYL (300 mg loading dose followed by
75 mg/day) or placebo, and were treated
for up to one year. Patients also
received aspirin (75–325 mg once daily)
and other standard therapies such as
heparin. The use of GPIIb/IIIa
inhibitors was not permitted for three
days prior to randomization.
The number of patients experiencing the
primary outcome (CV death, MI, or
stroke) was 582 (9.30%) in the
PLOGRYL-treated group and 719 (11.41%)
in the placebo-treated group, a 20%
relative risk reduction (95% CI of
10%–28%; p=0.00009) for the
PLOGRYL-treated group (see Table 3).
At the end of 12 months, the number of
patients experiencing the co-primary
outcome (CV death, MI, stroke or
refractory ischemia) was 1035 (16.54%)
in the PLOGRYL-treated group and 1187
(18.83%) in the placebo-treated group, a
14% relative risk reduction (95% CI of
6%–21%, p=0.0005) for the
PLOGRYL-treated group (see Table 3).
In the PLOGRYL-treated group, each
component of the two primary endpoints
(CV death, MI, stroke, refractory
ischemia) occurred less frequently than
in the placebo-treated group.
|
|
Table 3: Outcome Events in the CURE
Primary Analysis |
|
Outcome |
Plogryl
(+ aspirin) |
Placebo
(+ aspirin |
Relative Risk Reduction (%)
(95% CI) |
|
(n=6259) |
(n=6303) |
|
|
Other standard therapies were used
as appropriate
The individual
components do not represent a
breakdown of the primary and
co-primary outcomes, but rather the
total number of subjects
experiencing an event during the
course of the study. |
|
Primary outcome |
582 |
(9.3%) |
719 (11.4%) |
20% |
|
(Cardiovascular death, MI, Stroke) |
|
|
|
(10.3, 27.9) |
|
|
|
|
P=0.00009 |
|
Co-primary
outcome |
1035 |
(16.5%) |
1187 (18.8%) |
14% |
|
(Cardiovascular
death, MI, Stroke, Refractory
Ischemia) |
|
|
|
(6.2, 20.6) |
|
|
|
|
P=0.00052 |
|
All Individual
Outcome Events: |
|
CV death |
318 |
(5.1%) |
345 (5.5%) |
7%
(-7.7, 20.6) |
|
MI |
324 |
(5.2%) |
419 (6.6%) |
23%
(11.0, 33.4) |
|
Stroke |
75 |
(1.2%) |
87 (1.4%) |
14%
(-17.7, 36.6) |
|
Refractory
ischemia |
544 |
(8.7%) |
587 (9.3%) |
7%
(-4.0, 18.0) |
|
The benefits of PLOGRYL (clopidogrel
bisulfate) were maintained throughout
the course of the trial (up to 12
months). |
Figure 2: Cardiovascular Death,
Myocardial Infarction, and Stroke in the
CURE Study |
|
 |
|
In CURE, the use of PLOGRYL was
associated with a lower incidence of CV
death, MI or stroke in patient
populations with different
characteristics, as shown in Figure
3. The benefits associated with PLOGRYL
were independent of the use of other
acute and long-term cardiovascular
therapies, including heparin/LMWH (low
molecular weight heparin), IV
glycoprotein IIb/IIIa (GPIIb/IIIa)
inhibitors, lipid-lowering drugs,
beta-blockers, and ACE-inhibitors. The
efficacy of PLOGRYL was observed
independently of the dose of aspirin
(75–325 mg once daily). The use of oral
anticoagulants, non-study anti-platelet
drugs and chronic NSAIDs was not allowed
in CURE. |
Figure 3: Hazard Ratio for Patient
Baseline Characteristics and On-Study
Concomitant Medications/Interventions
for the CURE Study |
|
 |
|
The use of PLOGRYL in CURE was
associated with a decrease in the use of
thrombolytic therapy (71 patients [1.1%]
in the PLOGRYL group, 126 patients
[2.0%] in the placebo group; relative
risk reduction of 43%, P=0.0001), and
GPIIb/IIIa inhibitors (369 patients
[5.9%] in the PLOGRYL group, 454
patients [7.2%] in the placebo group,
relative risk reduction of 18%,
P=0.003). The use of PLOGRYL in CURE did
not impact the number of patients
treated with CABG or PCI (with or
without stenting), (2253 patients
[36.0%] in the PLOGRYL group, 2324
patients [36.9%] in the placebo group;
relative risk reduction of 4.0%,
P=0.1658).
In patients with ST-segment elevation
acute myocardial infarction, safety and
efficacy of clopidogrel have been
evaluated in two randomized,
placebo-controlled, double-blind
studies, COMMIT- a large outcome study
conducted in China - and CLARITY- a
supportive study of a surrogate endpoint
conducted internationally.
The randomized, double-blind,
placebo-controlled, 2×2 factorial design
COMMIT trial included 45,852 patients
presenting within 24 hours of the onset
of the symptoms of suspected myocardial
infarction with supporting ECG
abnormalities (i.e., ST elevation, ST
depression or left bundle-branch block).
Patients were randomized to receive
PLOGRYL (75 mg/day) or placebo, in
combination with aspirin (162 mg/day),
for 28 days or until hospital discharge
whichever came first.
The co-primary endpoints were death from
any cause and the first occurrence of
re-infarction, stroke or death.
The patient population included 28%
women, 58% patients ≥60 years (26%
patients ≥70 years) and 55% patients who
received thrombolytics, 68% received
ace-inhibitors, and only 3% had
percutaneous coronary intervention (PCI).
As shown in Table 4 and Figures 4 and 5
below, PLOGRYL significantly reduced the
relative risk of death from any cause by
7% (p = 0.029), and the relative risk of
the combination of re-infarction, stroke
or death by 9% (p = 0.002).
|
|
Table 4: Outcome
Events in the COMMIT Analysis |
|
Event |
PLOGRYL
(+ aspirin)
(N=22961) |
Placebo
(+ aspirin)
(N=22891) |
Odds ratio
(95% CI) |
p-value |
The difference
between the composite endpoint and
the sum of death+non-fatal MI+non-fatal
stroke indicates that 9 patients (2
clopidogrel and 7 placebo) suffered
both a non-fatal stroke and a
non-fatal MI.
Non-fatal MI and non-fatal stroke
exclude patients who died (of any
cause). |
Composite endpoint:
Death, MI, or Stroke |
2121 (9.2%) |
2310 (10.1%) |
0.91 (0.86, 0.97) |
0.002 |
|
Death |
1726 (7.5%) |
1845 (8.1%) |
0.93 (0.87, 0.99) |
0.029 |
|
Non-fatal MI |
270 (1.2%) |
330 (1.4%) |
0.81 (0.69, 0.95) |
0.011 |
|
Non-fatal Stroke |
127 (0.6%) |
142 (0.6%) |
0.89 (0.70, 1.13) |
0.33 |
|
All treated patients received aspirin.
|
|
Figure 4:
Cumulative Event Rates for Death in the
COMMIT Study |
|
 |
|
All treated patients
received aspirin.
|
Figure 5: Cumulative Event Rates for the
Combined Endpoint Re-Infarction, Stroke
or Death in the COMMIT Study |
|
 |
|
The effect of PLOGRYL did not differ
significantly in various pre-specified
subgroups as shown in Figure 6.
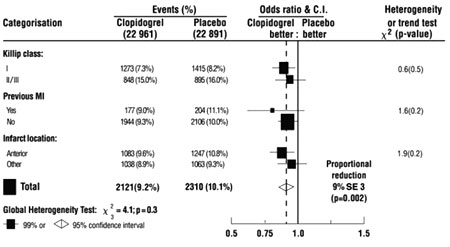
Additionally, the effect was similar in
non-prespecified subgroups including
those based on infarct location, Killip
class or prior MI history (see Figure
7). Such subgroup analyses should be
interpreted very cautiously. |
|
Figure 6: Effects of
Adding PLOGRYL to Aspirin on the
Combined Primary Endpoint across
Baseline and Concomitant Medication
Subgroups for the COMMIT Study |
|
 |
|
Three similar-sized prognostic index
groups were based on absolute risk of
primary composite outcome for each
patient calculated from baseline
prognostic variables (excluding
allocated treatments) with a Cox
regression model. |
Figure 7: Effects of
Adding PLOGRYL to Aspirin in the Non-Prespecified
Subgroups in the COMMIT Study |
|
 |
|
The randomized, double-blind,
placebo-controlled CLARITY trial
included 3,491 patients, 5% U.S.,
presenting within 12 hours of the onset
of a ST elevation myocardial infarction
and planned for thrombolytic therapy.
Patients were randomized to receive
PLOGRYL (300-mg loading dose, followed
by 75 mg/day) or placebo until
angiography, discharge, or Day 8.
Patients also received aspirin (150 to
325 mg as a loading dose, followed by 75
to 162 mg/day), a fibrinolytic agent
and, when appropriate, heparin for 48
hours. The patients were followed for 30
days
The primary endpoint was the occurrence
of the composite of an occluded
infarct-related artery (defined as TIMI
Flow Grade 0 or 1) on the predischarge
angiogram, or death or recurrent
myocardial infarction by the time of the
start of coronary angiography.
The patient population was mostly
Caucasian (89.5%) and included 19.7%
women and 29.2% patients ≥65 years. A
total of 99.7% of patients received
fibrinolytics (fibrin specific: 68.7%,
non-fibrin specific: 31.1%), 89.5%
heparin, 78.7% beta-blockers, 54.7% ACE
inhibitors and 63% statins.
The number of patients who reached the
primary endpoint was 262 (15.0%) in the
PLOGRYL-treated group and 377 (21.7%) in
the placebo group, but most of the
events related to the surrogate endpoint
of vessel patency.
|
|
Table 5: Event
Rates for the Primary Composite
Endpoint in the CLARITY Study |
| |
Clopidogrel
1752 |
Placebo
1739 |
OR |
95% CI |
|
The total number of patients with a
component event (occluded IRA,
death, or recurrent MI) is greater
than the number of patients with a
composite event because some
patients had more than a single type
of component event. |
|
Number (%) of
patients reporting the composite
endpoint |
262 (15.0%) |
377 (21.7%) |
0.64 |
0.53, 0.76 |
|
Occluded IRA |
|
|
|
|
|
|
|
|
|
|
N (subjects
undergoing angiography) |
1640 |
1634 |
|
|
|
n (%) patients
reporting endpoint |
192 (11.7%) |
301 (18.4%) |
0.59 |
0.48, 0.72 |
|
Death |
|
|
|
|
|
n (%) patients
reporting endpoint |
45 (2.6%) |
38 (2.2%) |
1.18 |
0.76, 1.83 |
|
Recurrent MI |
|
|
|
|
|
n (%) patients
reporting endpoint |
44 (2.5%) |
62 (3.6%) |
0.69 |
0.47, 1.02 |
|
|
INDICATIONS AND USAGE
:
PLOGRYL (clopidogrel bisulfate) is
indicated for the reduction of
atherothrombotic events as follows:
• Recent MI, Recent Stroke or
Established Peripheral Arterial Disease
For patients with a history of recent
myocardial infarction (MI), recent
stroke, or established peripheral
arterial disease, PLOGRYL has been shown
to reduce the rate of a combined
endpoint of new ischemic stroke (fatal
or not), new MI (fatal or not), and
other vascular death.
• Acute Coronary Syndrome
For patients with non-ST-segment
elevation acute coronary syndrome
(unstable angina/non-Q-wave MI)
including patients who are to be managed
medically and those who are to be
managed with percutaneous coronary
intervention (with or without stent) or
CABG, PLOGRYL has been shown to decrease
the rate of a combined endpoint of
cardiovascular death, MI, or stroke as
well as the rate of a combined endpoint
of cardiovascular death, MI, stroke, or
refractory ischemia.
For patients with ST-segment elevation
acute myocardial infarction, PLOGRYL has
been shown to reduce the rate of death
from any cause and the rate of a
combined endpoint of death,
re-infarction or stroke. This benefit is
not known to pertain to patients who
receive primary angioplasty
CONTRAINDICATIONS
:
The use of PLOGRYL is contraindicated in
the following conditions:
Hypersensitivity to the drug substance
or any component of the product.
Active pathological bleeding such as
peptic ulcer or intracranial hemorrhage.
WARNINGS :
Reduced effectiveness due to impaired
CYP2C19 function
The inhibition of platelet aggregation
by clopidogrel is entirely due to an
active metabolite. Clopidogrel is
metabolized to this active metabolite in
part by CYP2C19. This metabolism can be
impaired by genetic variations in
CYP2C19 and by concomitant medications
that interfere with CYP2C19. Avoid use
of PLOGRYL in patients with impaired
CYP2C19 function due to known genetic
variation or due to drugs that inhibit
CYP2C19 activity.
Genetic variations :
Patients with genetically reduced
CYP2C19 function have diminished
antiplatelet responses and generally
exhibit higher cardiovascular event
rates following myocardial infarction
than do patients with normal CYP2C19
function
Drug interactions :
Co-administration of PLOGRYL with
omeprazole, a proton pump inhibitor that
is an inhibitor of CYP2C19, reduces the
pharmacological activity of PLOGRYL if
given concomitantly or if given 12 hours
apart. There is no evidence that other
drugs that reduce stomach acid, such as
most H2 blockers (except cimetidine,
which is a CYP2C19 inhibitor) or
antacids interfere with the antiplatelet
activity of clopidogrel
Thrombotic thrombocytopenic purpura (TTP)
:
TTP has been reported rarely following
use of PLOGRYL, sometimes after a short
exposure (<2 weeks). TTP is a serious
condition that can be fatal and requires
urgent treatment including
plasmapheresis (plasma exchange). It is
characterized by thrombocytopenia,
microangiopathic hemolytic anemia (schistocytes
[fragmented RBCs] seen on peripheral
smear), neurological findings, renal
dysfunction, and fever.
PRECAUTIONS :
General: PLOGRYL prolongs the bleeding
time and therefore should be used with
caution in patients who may be at risk
of increased bleeding from trauma,
surgery, or other pathological
conditions (particularly
gastrointestinal and intraocular). If a
patient is to undergo elective surgery
and an antiplatelet effect is not
desired, PLOGRYL should be discontinued
5 days prior to surgery.
Due to the risk of bleeding and
undesirable hematological effects, blood
cell count determination and/or other
appropriate testing should be promptly
considered, whenever such suspected
clinical symptoms arise during the
course of treatment
In patients with recent TIA or stroke
who are at high risk of recurrent
ischemic events, the combination of
aspirin and PLOGRYL has not been shown
to be more effective than PLOGRYL alone,
but the combination has been shown to
increase major bleeding.
GI Bleeding: In CAPRIE, PLOGRYL was
associated with a rate of
gastrointestinal bleeding of 2.0%, vs.
2.7% on aspirin. In CURE, the incidence
of major gastrointestinal bleeding was
1.3% vs. 0.7% (PLOGRYL + aspirin vs.
placebo + aspirin, respectively).
PLOGRYL should be used with caution in
patients who have lesions with a
propensity to bleed (such as ulcers).
Drugs that might induce such lesions
should be used with caution in patients
taking PLOGRYL.
Use in Hepatically-Impaired Patients:
Experience is limited in patients with
severe hepatic disease, who may have
bleeding diatheses. PLOGRYL should be
used with caution in this population.
Use in Renally-Impaired Patients:
Experience is limited in patients with
severe renal impairment. PLOGRYL should
be used with caution in this population.
Information for Patients :
Patients should be told that while
taking PLOGRYL or PLOGRYL combined with
aspirin:
• it may take them longer than usual to
stop bleeding;
• they may bruise and/or bleed more
easily;
• they should report any unusual
bleeding to their physician;
• they should tell their physician about
any other medications they are taking,
including prescription or
over-the-counter omeprazole;
• they should inform physicians and
dentists that they are taking PLOGRYL
and/or any other product known to affect
bleeding before any surgery is scheduled
and before any new drug is taken.
Drug Interactions :
Clopidogrel is metabolized to its active
metabolite in part by CYP2C19.
Concomitant use of drugs that inhibit
the activity of this enzyme results in
reduced plasma concentrations of the
active metabolite of clopidogrel and a
reduction in platelet inhibition. Avoid
concomitant use of drugs that inhibit
CYP2C19, including omeprazole,
esomeprazole, cimetidine, fluconazole,
ketoconazole, voriconazole, etravirine,
felbamate, fluoxetine, fluvoxamine, and
ticlopidine (see WARNINGS)
Omeprazole :
In a crossover clinical study, 72
healthy subjects were administered
PLOGRYL (300-mg loading dose followed by
75 mg/day) alone and with omeprazole (80
mg at the same time as PLOGRYL) for 5
days.
The exposure to the active metabolite of
clopidogrel was decreased by 46% (Day 1)
and 42% (Day 5) when PLOGRYL and
omeprazole were administered together.
Mean inhibition of platelet aggregation
(IPA) was diminished by 47% (24 hours)
and 30% (Day 5) when PLOGRYL and
omeprazole were administered together.
In another study 72 healthy subjects
were given the same doses of PLOGRYL and
omeprazole but the drugs were
administered 12 hours apart; the results
were similar indicating that
administering PLOGRYL and omeprazole at
different times does not prevent their
interaction (see WARNINGS).
Aspirin :
Aspirin did not modify the clopidogrel-mediated
inhibition of ADP-induced platelet
aggregation. Concomitant administration
of 500 mg of aspirin twice a day for 1
day did not significantly increase the
prolongation of bleeding time induced by
PLOGRYL. PLOGRYL potentiated the effect
of aspirin on collagen-induced platelet
aggregation. PLOGRYL and aspirin have
been administered together for up to one
year.
Heparin :
In a study in healthy volunteers,
PLOGRYL did not necessitate modification
of the heparin dose or alter the effect
of heparin on coagulation.
Coadministration of heparin had no
effect on inhibition of platelet
aggregation induced by PLOGRYL
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
:
In healthy volunteers receiving
naproxen, concomitant administration of
PLOGRYL was associated with increased
occult gastrointestinal blood loss.
NSAIDs and PLOGRYL should be
coadministered with caution.
Warfarin :
Because of the increased risk of
bleeding, the concomitant administration
of warfarin with PLOGRYL should be
undertaken with caution. (See
PRECAUTIONS: General.)
Other Concomitant Therapy :
No clinically significant
pharmacodynamic interactions were
observed when PLOGRYL was coadministered
with atenolol, nifedipine, or both
atenolol and nifedipine. The
pharmacodynamic activity of PLOGRYL was
also not significantly influenced by the
coadministration of phenobarbital or
estrogen.
The pharmacokinetics of digoxin or
theophylline were not modified by the
coadministration of PLOGRYL (clopidogrel
bisulfate).
At high concentrations in vitro,
clopidogrel inhibits P450 (2C9).
Accordingly, PLOGRYL may interfere with
the metabolism of phenytoin, tamoxifen,
tolbutamide, warfarin, torsemide,
fluvastatin, and many non-steroidal
anti-inflammatory agents, but there are
no data with which to predict the
magnitude of these interactions. Caution
should be used when any of these drugs
is coadministered with PLOGRYL.
In addition to the above specific
interaction studies, patients entered
into clinical trials with PLOGRYL
received a variety of concomitant
medications including diuretics,
beta-blocking agents, angiotensin
converting enzyme inhibitors, calcium
antagonists, cholesterol lowering
agents, coronary vasodilators,
antidiabetic agents (including insulin),
thrombolytics, heparins (unfractionated
and LMWH), GPIIb/IIIa antagonists,
antiepileptic agents and hormone
replacement therapy without evidence of
clinically significant adverse
interactions.
There are no data on the concomitant use
of oral anticoagulants, non study oral
anti-platelet drugs and chronic NSAIDs
with clopidogrel.
Carcinogenesis, Mutagenesis, Impairment
of Fertility :
There was no evidence of tumorigenicity
when clopidogrel was administered for 78
weeks to mice and 104 weeks to rats at
dosages up to 77 mg/kg per day, which
afforded plasma exposures >25 times that
in humans at the recommended daily dose
of 75 mg.
Clopidogrel was not genotoxic in four in
vitro tests (Ames test, DNA-repair test
in rat hepatocytes, gene mutation assay
in Chinese hamster fibroblasts, and
metaphase chromosome analysis of human
lymphocytes) and in one in vivo test
(micronucleus test by oral route in
mice).
Clopidogrel was found to have no effect
on fertility of male and female rats at
oral doses up to 400 mg/kg per day (52
times the recommended human dose on a
mg/m2 basis).
Pregnancy
Pregnancy Category B :
Reproduction studies performed in rats
and rabbits at doses up to 500 and 300
mg/kg/day (respectively, 65 and 78 times
the recommended daily human dose on a
mg/m2 basis), revealed no evidence of
impaired fertility or fetotoxicity due
to clopidogrel. There are, however, no
adequate and well-controlled studies in
pregnant women. Because animal
reproduction studies are not always
predictive of a human response, PLOGRYL
should be used during pregnancy only if
clearly needed.
Nursing Mothers :
Studies in rats have shown that
clopidogrel and/or its metabolites are
excreted in the milk. It is not known
whether this drug is excreted in human
milk. Because many drugs are excreted in
human milk and because of the potential
for serious adverse reactions in nursing
infants, a decision should be made
whether to discontinue nursing or to
discontinue the drug, taking into
account the importance of the drug to
the nursing woman.
Pediatric Use :
Safety and effectiveness in the
pediatric population have not been
established.
Geriatric Use:
Of the total number of subjects in the
CAPRIE, CURE and CLARITY controlled
clinical studies, approximately 50% of
patients treated with PLOGRYL were 65
years of age and older, and 15% were 75
years and older. In COMMIT,
approximately 58% of the patients
treated with PLOGRYL were 60 years and
older, 26% of whom were 70 years and
older.
The observed risk of thrombotic events
with clopidogrel plus aspirin versus
placebo plus aspirin by age category is
provided in Figures 3 and 6 for the CURE
and COMMIT trials, respectively (see
CLINICAL STUDIES). The observed risk of
bleeding events with clopidogrel plus
aspirin versus placebo plus aspirin by
age category is provided in Tables 6 and
7 for the CURE and COMMIT trials,
respectively (see ADVERSE REACTIONS).
ADVERSE REACTIONS :
PLOGRYL has been evaluated for safety in
more than 42,000 patients, including
over 9,000 patients treated for 1 year
or more. The clinically important
adverse events observed in CAPRIE, CURE,
CLARITY and COMMIT are discussed below.
The overall tolerability of PLOGRYL in
CAPRIE was similar to that of aspirin
regardless of age, gender and race, with
an approximately equal incidence (13%)
of patients withdrawing from treatment
because of adverse reactions.
Hemorrhagic :
In CAPRIE patients receiving PLOGRYL,
gastrointestinal hemorrhage occurred at
a rate of 2.0%, and required
hospitalization in 0.7%. In patients
receiving aspirin, the corresponding
rates were 2.7% and 1.1%, respectively.
The incidence of intracranial hemorrhage
was 0.4% for PLOGRYL compared to 0.5%
for aspirin.
In CURE, PLOGRYL use with aspirin was
associated with an increase in bleeding
compared to placebo with aspirin (see
Table 6). There was an excess in major
bleeding in patients receiving PLOGRYL
plus aspirin compared with placebo plus
aspirin, primarily gastrointestinal and
at puncture sites. The incidence of
intracranial hemorrhage (0.1%), and
fatal bleeding (0.2%), were the same in
both groups.
The overall incidence of bleeding is
described in Table 6 for patients
receiving both
PLOGRYL and aspirin in CURE.
|
|
Wallpapers :


CLICK ON PHOTO TO SEE LARGE.


|
|
|
|
|
 |
|
|
|
